Carbonic Acid Strong or Weak
PH log 10 H. H2CO3 is a weak acid that dissociates into a proton H cation and a bicarbonate ion HCO3- anion.
Why Is Carbonic Acid A Weak Acid Even Though It Gets Completely Dissociated Into H And Co3 Ions Quora
Every substance will have its own Ka value.
. Students can get a complete understanding of this topic on the official site of Vedantu where the experts have. Strong acid will have the different characteristic with the weak acid. It will have the negative value of Ka.
LAGUNA DESIGN Getty Images. On the other hand a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. A weak acid or a weak base only partially dissociates.
They are useful across the entire pH range. StrongWeak Acids Bases When a strong acidbase dissolves in water nearly all of the acidbase molecules will dissociate into ions The greater the ability to dissociate the more potential the acid or base has for being dangerous because there are more ions available to react When a weak acidbase dissolves in water only a small fraction of the acidbase molecules. All new derivatives S1S22 were assayed against human carbonic anhydrase hCAs IX and XII for their inhibitory.
Furthermore the conjugate base of carbonic acid which is the bicarbonate ion is a relatively good base. They exhibit a high capacity for the alkaline earth metals associated with alkalinity and a more limited capacity for the alkali metals. When sodium carbonate is dissolved in water it is partially hydrolyzed yielding sodium hydroxide and carbonic acid.
CH 2 O 3 Also known as. At equilibrium both the acid and the conjugate base are present in solution. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table.
The examples of strong acids are sulfuric acid H2SO4 perchloric. HCl H 2 O H 3 O Cl When the two solutions are mixed the H. Since the Ka is quite small acetic acid is a weak acid and it does not completely ionize in water.
As a result the aqueous solution is pH neutral. The terms molecule compound and atom can be confusing. The simple question What is.
Aerial acid acid of air dihydrogen carbonate kihydroxyketone. These are the reasons why carbonic. Certain weak-base resins acrylic can also reduce carbonic acid.
This compound only partly dissociates in aqueous solutions. A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion. Only a small fraction of the acid molecule.
Bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Heres an explanation of what a molecule is with some examples of common molecules. Nonetheless there can be some exceptions as Hydrofluoric acids p H is 327 which is also low as strong acid hydrochloric acid with a pH value of 301.
AH H 2 O A-aq H 3 O aq. This is the only acid excreted by the lungs as a gas. Weak-base resins as a rule do not remove weak acids such as carbonic or silicic but will neutralize strong acids nitric sulfuric and hydrochloric.
When dissolved in water the strong base sodium hydroxide ionizes into hydroxide and sodium ions. Ka itself is the equilibrium constant for the acid dissoleve reaction in the water. Example Is Acetic Acid Strong or Weak.
Acid with values less than one are considered weak. Carbonic acid is a weak. Resin capacity therefore has to be defined in terms of strong-acidweak-acid and strong-baseweak-base functions.
This is the chemical structure of carbonic acid. Characterized by their ability to exchange cations or split neutral salts. A Dilute hydrochloric acid b Dilute NaOH solution c Dilute ethanoic acid solution d Lemon juice e Water f Dilute sodium bicarbonate solution Theory pH is the measure of the hydrogen ion concentration H of a solution.
Acetic acid has a Ka value of 18 x 10-5. Very little research has been done on sparkling water in particular but much more has been done on other. The strong acid has the tendency to fully dissolve in the water solution.
Examples of strong acids and bases are given in the table below. In aqueous solution each of these essentially ionizes 100. In this study new sulphamethoxazole derivatives S1S4 S6S12 and S14S22 were designed and synthesized and their structures were fully characterized and validated using NMR mass and IR spectroscopy as well as elemental analyses.
Solutions of carbon dioxide in water carbonated water may be called carbonic acid. NH 23 CO sl HO 2 2NaOH aq HC 23 O aq Here NaOH releases OH. No carbonic acid is not a strong acid.
Sodium hydroxide is now a strong base that is fully ionised and produces a significant number of hydroxide ions OH aq. Weak acids remain mainly as a complete molecule in solution. Aim To find the pH of the following samples by using pH paperuniversal indicator.
Here hydrochloric acid strong acid ionises to give Haq ions which is greater than ammonium hydroxide weak base ionises to give OH-aq ions so the solution is acidic. Its pH value is less than that of strong acids. The salt of a strong base NaOH and a w eak acid HC 23 O gives basic solution pH more than 7.
Have a high affinity for the hydrogen ion and are therefore easily regenerated with strong acids. Examples of Weak Acids and Bases List of Weak Acids. NaOH Na OH and similarly in water the acid hydrogen chloride forms hydronium and chloride ions.
Surely any acid even a weak one is going to erode the enamel on our teeth. The value of pH for a weak acid is less than 7 and not neutral 7. Ph Value Experiment Class 10 CBSE.
Carbonic acid on the other hand is a weak acid.

Is Carbonic Acid Is A Strong Electrolyte Quora

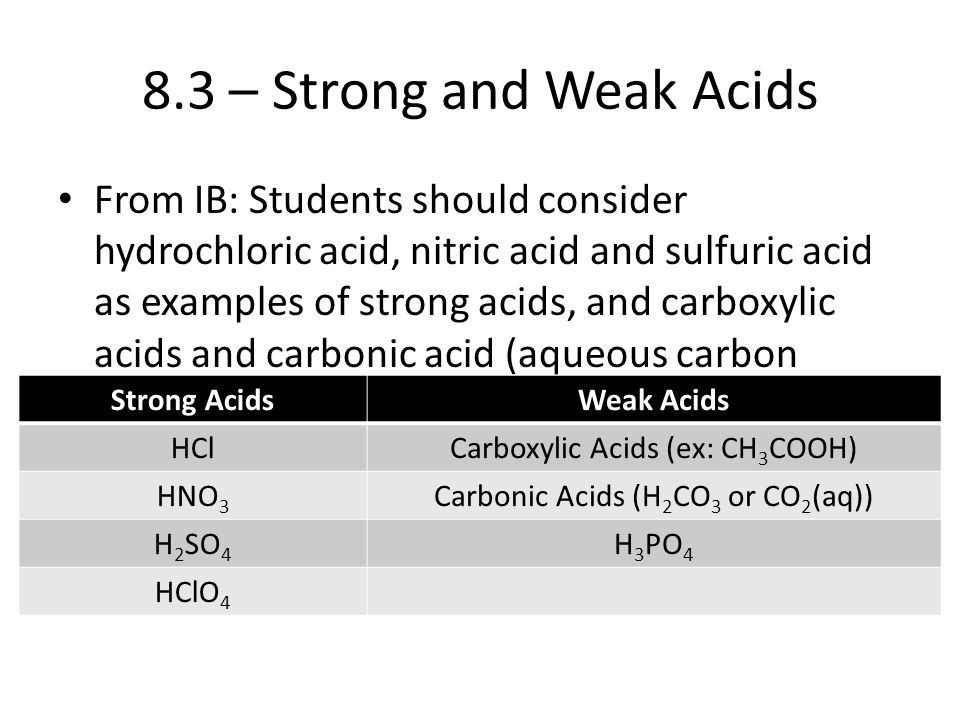
Topic 08 Acids Bases 8 3 Strong And Weak Acids And Bases Ppt Download

Is H2co3 An Acid Or Base Or Both Strong Or Weak Carbonic Acid

Acids And Bases Teaching Chemistry Chemistry Review Study Notes
0 Response to "Carbonic Acid Strong or Weak"
Post a Comment